Flow cytometric analysis of Ki-67 expression in canine cutaneous mast cell tumours and its prognostic value
12 February 2026
Mast cell tumours (MCTs) are the most commonly diagnosed cutaneous tumours in dogs, accounting for around 16–21% of all cutaneous neoplasms. While histopathologic grading remains the most reliable prognostic tool, it is not infallible. There are well-differentiated or intermediate-grade MCTs that still lead to death in affected dogs, highlighting a need for additional, more precise prognostic indicators such as assessment of Ki-67 index.
This clinical challenge motivated our project: to explore whether flow cytometry on fine needle aspirates could be used to assess key prognostic biomarkers, particularly Ki-67 expression, in a minimally invasive and pre-operative manner.
Understanding the markers: Ki-67 and c-KIT
Ki-67 is a nuclear protein associated with cell proliferation. It is present in all active phases of the cell cycle and absent in resting cells, making it an excellent marker of cell division. In MCTs, Ki-67 expression has been shown to correlate with survival outcomes. Currently, Ki-67 is evaluated via immunohistochemistry (IHC) on tumour samples obtained surgically. This approach can delay prognostication until after a major clinical decision (such as surgical excision) has already been made.
Another established prognostic factor is the mitotic count, which also reflects proliferative activity. However, studies suggest only a moderate correlation between Ki-67 and mitotic index. Interestingly, a high mitotic index reliably predicts poor outcomes, but a low mitotic index does not always mean the tumour will behave benignly. Conversely, low Ki-67 expression is a strong indicator of favourable prognosis, but high Ki-67 should not automatically be considered a poor prognostic sign.
In addition to Ki-67, c-KIT (CD117) expression patterns have also been investigated. c-KIT is a membrane receptor tyrosine kinase, and its dysregulation has been implicated in the pathogenesis and behaviour of MCTs. IHC studies have identified three c-KIT staining patterns: membrane-associated, focal/stippled cytoplasmic, and diffuse cytoplasmic. The latter two have been associated with more aggressive biological behaviour.
Given the increasing clinical use of non-surgical treatments—such as intratumoural injection of tigilanol tiglate, which destroys tumour tissue without histological sampling—developing reliable, minimally invasive methods of assessing prognosis is more relevant than ever.
Key aims of the research and early findings
Our primary objective was to determine whether Ki-67 expression could be accurately measured using flow cytometry on fine needle aspirates of MCTs and whether those measurements would correlate with Ki-67 levels determined by conventional IHC.
We also aimed to explore whether imaging flow cytometry could help characterise c-KIT expression patterns, potentially enabling us to build a broader prognostic panel from minimally invasive samples. This would be particularly helpful in clinical scenarios where tumour excision is not possible or desired.
Immunostaining for both Ki-67 and c-KIT was carried out following published techniques. Ki-67 quantification was initially performed manually by two board-certified anatomic pathologists. As the project progressed, we developed and implemented an automated quantification method using the QuPath digital pathology platform.
While our full analysis is ongoing and will be submitted for peer-reviewed publication, preliminary data are encouraging. Our flow cytometry approach shows potential in identifying Ki-67 expression in mast cell tumour cells, and early comparisons with IHC results appear promising (Figure 1 A-C).
We hope that once validated, this method could allow faster, less invasive prognostication—potentially available before surgery or other treatments. This could be particularly valuable when considering tigilanol tiglate treatment, managing elderly or fragile patients, or helping clients make informed treatment choices early in the diagnostic process.
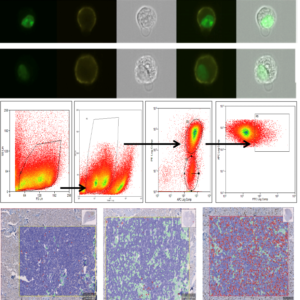
Figure 1A Imaging flow cytometry with green, nuclear Ki-67 staining and yellow, surface staining for c-KIT. Figure 1B Flow cytometry data obtained from a study sample. Figure 1C Ki-67 IHC analysis on histological sections via QuPath, distinguishing between tumour cells (Ki-67+ in red, Ki-67– in blue) and tumour stroma (green).
Impact on veterinary practice
We believe this research may significantly impact both general and referral practice. The ability to estimate tumour behaviour from a simple fine needle sample could transform how staging and treatment planning are approached.
Although clinical adoption is likely a few years away—pending further validation—we are hopeful that practices will soon be able to submit samples for analysis and receive timely, meaningful, prognostic data. This would reduce the need for invasive procedures and offer vets and owners more flexibility and confidence in managing canine MCTs.
The personal research experience
I find oncology fascinating not only because of the strong bonds we build with our patients and clients, but also because it remains a rapidly evolving field with enormous potential for research-driven improvement. Despite considerable advances in veterinary oncology, we are still far from curing most cancers, and there is ample room to improve diagnostics, prognostication, and treatment protocols for veterinary cancer patients.
This research project has been a true learning curve—teaching me how to apply for funding, design studies, collaborate across disciplines, and bring a clinical question to life through structured investigation. Working with experienced researchers and scientists at the University of Cambridge has been a privilege. It’s also been personally enriching, reinforcing my passion for oncology and research.
The pilot phase was both challenging and rewarding. Building a collaborative team, developing new lab methods, and translating a clinical problem into a research question was a unique and exciting experience.
We encountered several obstacles: financial limitations (overcome thanks to the PetSavers grant), technical difficulties with optimising flow cytometry protocols, and the challenge of acquiring enough suitable clinical samples. Nonetheless, the project has been deeply fulfilling. One of the highlights has been engaging with local practices who kindly contributed samples and participated in CPD sessions we delivered on mast cell tumours.
Thinking of applying for a BSAVA PetSavers research grant?
The support from BSAVA PetSavers has been essential—not only in funding but also in providing visibility and legitimacy to our project. Their involvement throughout has helped shape the project and offered opportunities to share our findings with the wider veterinary community.
If you are a vet or nurse in either general or referral practice and have an idea or a clinical problem, you’re passionate about, I encourage you to apply. You don’t need to be in a research-heavy environment—just curious, motivated, and willing to collaborate. PetSavers can provide the support to help you take that first step.
About the author
Petros Odatzoglou graduated from the University of Thessaly, Greece, in 2017. His interest in oncology began early in his veterinary studies, driven by a noticeable lack of oncology specialists in Greece and his personal experience of losing a beloved dog to cancer. After graduation, he moved to the UK and completed a rotating internship at Vale Referrals, followed by another at Fitzpatrick Referrals Oncology and Soft Tissue Hospital (now Aura). He then spent time in general practice while also pursuing an MSc in Cancer and Clinical Oncology at the Barts Cancer Institute, Queen Mary University of London.
In 2021, he joined the Queen’s Veterinary School Hospital at the University of Cambridge as a Junior Clinical Training Scholar in Oncology. The following year, he started his ECVIM-CA residency (Senior Clinical Training Scholar), which he completed in August 2025. In parallel, he obtained his MSc with distinction.
This project, funded by BSAVA PetSavers, has formed the core research component of his residency training. He has had the opportunity to work alongside a fantastic team: Rachel Hewitt, Cassia Hare, Katherine Hughes, Hannah Wong, Barbara Blacklaws, and Jane Dobson, all based at the Department of Veterinary Medicine, University of Cambridge. Their backgrounds in immunology and flow cytometry, diagnostic histology and oncology are all key to the project.

