Feline chronic inflammatory hepatobiliary disease
19 April 2024
Dr Penny Watson at the University of Cambridge received PetSavers funding for a master’s degree to investigate markers for chronic biliary tract disease in cats. The master’s student, Jason Bestwick, used a bespoke hepatic histological scoring system to search for serum autoantibodies in an attempt to better define the disease aetiology.
Feline cholangitis has been a well-recognized condition since the 1970/80s. The nomenclature used to describe forms of the disease has varied but, traditionally, histological findings have been used as defining features. In 2006, the WSAVA Liver Standardization Group detailed four sub-categories of feline cholangitis: neutrophilic, lymphocytic, destructive and chronic (associated with liver fluke infestation).1
More recently, the term cholangitis/cholangiohepatitis syndrome (CCHS), split into suppurative (S-CCHS) and non-suppurative (NS-CCHS), has been suggested to refer to neutrophilic and lymphocytic cholangitis, respectively.2,3 Based on available histopathological surveys,4 S-CCHS/neutrophilic and NS-CCHS/lymphocytic cholangitis are the most common types of cholangitis diagnosed in UK cats. S-CCHS is typically attributed to ascending bacterial infections whilst NS-CCHS is of unknown aetiology but is suspected to be autoimmune/immune-mediated, based on its histological appearance and possible response to immunosuppression.
In the human field of hepatology, as in veterinary medicine, liver disease can be classified on the basis of aetiology (e.g. autoimmune/immune-mediated, infectious, hereditary, obesity- and alcohol-related) and by anatomical location (e.g. parenchymal disease, gallbladder disease, disease of the biliary system [cholangiopathies]). Focusing on aetiology and the anatomical location of disease, immune-mediated cholangiopathies in humans comprise three major diseases/ syndromes: primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC) and IgG4-associated cholangitis.
PSC and PBC are both suspected immune-mediated cholangiopathies, the former affecting the intra- and extra-hepatic bile ducts of humans, and the latter affecting the intra-hepatic bile ducts.5,6 PSC can affect people of any age with a 60% male:40% female split, with approximately 80% patients having concurrent inflammatory bowel disease (IBD). PBC predominantly affects middle-aged females (10% male:90% female) and concurrent IBD is rare. For PSC, the typical route to diagnosis is through magnetic resonance cholangiopancreatography (demonstrating segmental strictures and dilations of the biliary tract) and the exclusion of secondary causes of sclerosing cholangitis (such as toxic and infectious disease). Liver histology is not pathognomonic (mild lymphocytic portal inflammation); however, concentric periductal fibrosis is the characteristic histologic feature. There is currently no known effective medical treatment and liver transplantation is indicated in patients with end-stage disease. The diagnosis of PBC is made when two out of three of the following criteria are met: anti-mitochondrial antibody positivity, elevated liver enzyme activity and diagnostic liver biopsy. Liver histology has a characteristic, but not pathognomonic, appearance (focal granulomatous and lymphocytic portal inflammation); cirrhosis is seen in the late stages of the disease. The medical treatment of choice in ursodeoxycholic acid, which is associated with prolonged liver transplant-free survival time.
As exemplified above, these diseases are not diagnosed purely on histology (which may be non-specific) but on a combination of appropriate signalment, typical blood-work findings and the presence and pattern of autoantibodies.8 Indirect immunofluorescence (IFF) is the primary method used for the detection of these autoantibodies, where the use of various tissue- and cell-types to bind and detect them enables inferences about their molecular targets (Figure 1).
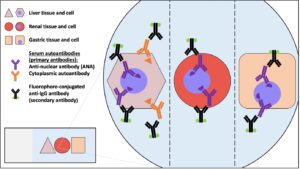
Figure 1. Principle for the detection of serum autoantibodies using indirect immunofluorescence. Standard procedures use triple rodent tissue as a substrate for autoantibody binding. Human patient serum is incubated on sections of rodent liver, kidney and stomach and any bound autoantibodies are detected using secondary antibodies against human IgG that are labelled with a fluorescent marker. An immortalized human epithelial cell line, HEp2, are also used as a substrate for the binding and detection of serum autoantibodies. Reproduced from Bestwick JP, MPhil thesis, University of Cambridge, 2023.
The objective of the current project was to explore if a sub-population of cats with chronic biliary disease might have serum autoantibodies, supporting an immune-mediated aetiology, and also the possible existence of multiple disease syndromes within the CCHS complex. Additionally, by concisely describing the histological features of the cats’ livers, we aimed to explore if specific findings and their severity might be associated with the presence of autoantibodies.
Methods
As part of the project, we devised a bespoke combination of modified, previously used, histological scoring systems (feline and human) to examine and succinctly describe the liver histopathology of cats with various degrees of chronic biliary inflammation. This included examining the nature of and scoring the severity of inflammation, fibrosis and cholangiolar proliferation. Secondly, using archived serum from the same cats, we evaluated the presence of autoantibodies with IFF using a normal feline liver substrate. This tissue was obtained, with informed consent from the owners, from a formerly healthy cat euthanased as a result of traumatic injuries. Previously, only a single study has reported on autoantibodies in cats with liver disease, and the method of IIF (including the substrate used) was not described.9 The attempted detection of autoantibodies in other feline diseases has used variable substrates that are inconsistently feline- or disease/tissue-specific.10-14 By selecting a species- and disease/tissue-specific tissue (i.e. normal cat liver) as the IIF substrate for this project, it was hoped the probability of detected autoantibodies being liver-specific would be increased.
Results
Key findings from this work included:
■ Serum autoantibodies were detected in 7/34 cats with chronic inflammatory biliary disease whilst they were not detected in any control cat (0/12; cats without inflammatory hepatobiliary changes as documented on liver histology). – Two distinct patterns of fluorescence were recognized using IIF: nuclear (i.e. antinuclear antibodies; 4/7, Figure 2A) and cytoplasmic (3/7, Figure 2B).
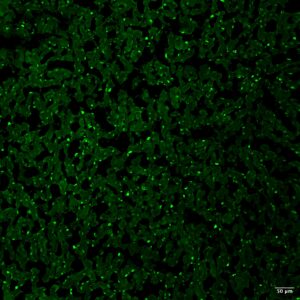
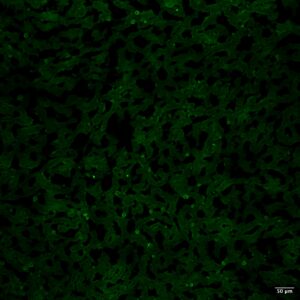
Figure 2. IFF for serum autoantibodies using cat liver as a substrate; magnification 10×; scale bar, 50 µm. (A) Positive nuclear staining. (B) Positive cytoplasmic staining.
■ Concentric periductal fibrosis, a typical finding in humans with PSC, was identified in the liver histology of 7/34 cats with chronic inflammatory biliary disease (Figure 3).
■ Regarding the portal lymphocyte population of cats with chronic inflammatory biliary disease, variable T-cell/B-cell predominance was found. This is contrary to previous findings of mostly T-cell predominance in cats with NS-CCHS.15,16
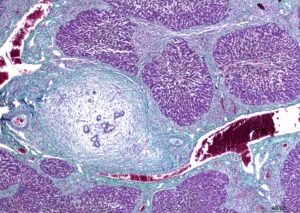
Figure 3. Liver histology from a cat showing severe (grade 3) bridging fibrosis and periductal concentric fibrosis. Stained with Masson’s trichrome; magnification 4×; scale bar, 400 µm.
Discussion
This preliminary work has given some clues to the presence of autoimmune disease in a subset of cats with CCHS. It also provided a histological scoring system that could be utilized in the future to uniformly describe hepatobiliary inflammation, fibrosis and cholangiolar proliferation in cats, and a methodology to detect autoantibodies in cats. It is hoped that continuation of this work might drive increased standardization of the histological assessment of feline hepatobiliary inflammation, discern a diagnostic utility of the detection of autoantibodies in cats with CCHS, and ultimately lead to the detection of multiple syndromes within CCHS. Subsequently, clinicians might then be able to more accurately determine treatment (e.g. which patients should receive immunosuppressives) and prognosis for cats with CCHS.
Funding acknowledgments and the research experience
This research was conducted through an MPhil at the University of Cambridge funded by BSAVA PetSavers. The project was supervised by Penny Watson, Fernando Constantino-Casas and Rachel Hewitt, all of whom shared their knowledge and expertise unreservedly. Without the support of BSAVA PetSavers and the project supervisors, this work would not have been possible. On a personal level, I will be forever grateful for the chance to pursue work for which I have great passion, the opportunity to learn new skills and for the encouragement and confidence the experience gave me to continue in research. To anyone considering applying directly for BSAVA PetSavers funding, or a BSAVA PetSavers-funded project, I would strongly recommend it!
About the authors
Jason Bestwick graduated from the University of Nottingham in 2012 and spent 3 years working in small animal general practice before completing a small animal internal medicine residency at the Animal Health Trust. He became a diplomate of the American College of Veterinary Internal Medicine in 2021. Following a longstanding interest in feline medicine, Jason commenced work on the research described within this article in October 2021, as part of a year-long BSAVA PetSavers-funded MPhil at the University of Cambridge. He is currently a PhD student at the Royal Veterinary College focusing on feline chronic kidney disease-mineral and bone disorder.
Penny Watson graduated from the University of Cambridge and is a diplomate of the European College of Veterinary Internal Medicine and Fellow of the Royal College of Veterinary Surgeons. Until her retirement in 2022, she held the position of Associate Professor in Small Animal Medicine at the University of Cambridge, where she spent much of her career focussed on the clinical management and scientific investigation of chronic liver and pancreas disease in both dogs and cats. She remains actively involved in the research of, and lecturing on, these topics. Penny was formerly Honorary Secretary of BSAVA and in 2017 was awarded the BSAVA Woodrow Award for outstanding contributions in the field of small animal veterinary medicine. She has been the recipient of several BSAVA PetSavers grants that have enabled numerous studies, including the one reported within this article.
