Endothelial dysfunction: an alternative perspective on canine myxomatous mitral valve disease
12 November 2024
Marco Mazzarella, Geoff Culshaw (Royal (Dick) School of Veterinary Studies) and their collaborator Natalie Jones (Queen’s Medical Research Institute) from the University of Edinburgh, were awarded BSAVA PetSavers funding in 2021 to study endothelial dysfunction in canine myxomatous mitral valve disease.
Myxomatous mitral valve disease (MMVD) is well known as the most common heart disease in dogs.1 Despite major successes over the last two decades in its treatment and management, it remains a debilitating condition. Further advances will require identification of novel therapeutic targets, but this is hindered by our lack of understanding of MMVD’s pathophysiology.
In humans, acquired cardiac disease is strongly associated with endothelial dysfunction,2 which is a deficiency in the ability of blood vessels (usually arteries) to dilate and constrict according to the needs of the local tissues they supply. There is good evidence that endothelial dysfunction occurs in canine MMVD,3,4 but the gold standard assessment of endothelial function, called isometric myography, has never been applied before to major arteries of pet dogs.
What will be the benefits of research?
Endothelial dysfunction is implicated in a lot of the morbidity associated with cardiac disease. This includes non-specific signs of loss of wellness, such as lethargy, weakness and inappetence, but also more major co-morbidities such as renal dysfunction (cardiorenal syndrome) and pancreatitis. Isometric myography (Figures 1 and 2)5 is an ex vivo method, which means it is performed on ‘living’ viable arteries that are removed after a dog has died. It measures the pharmacological response of the endothelium to different vasoactive substances6 when the artery is stretched to a pre-defined amount. In particular, it determines how well arteries dilate in response to locally produced nitric oxide; therefore, our research should provide insights into the amount of endothelial dysfunction present in MMVD, and which vasoactive substances might help arteries dilate in vivo. In so doing, we hope our research could provide the basis to new therapies that allow arteries to meet the demands of individual tissues, and ameliorate morbidity associated with MMVD.
|
FIGURE 1: Multiwire Myograph System 610 M (DMT) at The Roslin Institute, with four wells that can be equipped with pins or jaws to mount sections of arteries. |
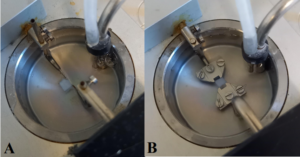
FIGURE 2: Wells with arteries mounted on pins (A) and wires (B). The pale colour of the arteries is typical after they have been collected and preserved. The bubbles seen in the medium come from the more internal tube, which is connected to a gas cylinder that maintains an infusion of O2/CO2 into the medium. Behind this is another tube connected to a vacuum system that removes the medium via the opening of a valve. Grease seen on the attachment of the pins and jaws is used for lubricating them. |
What are the aims of this research?
The aims of our study are to apply isometric myography to arteries taken from pet dogs after death, validate the technique and determine its feasibility. We also want to identify whether endothelial function varies between arteries taken from different sites, and whether dysfunction occurs with worsening MMVD.
What have we learned so far?
We have collected arterial samples from dogs that were euthanased for medical and welfare reasons and which were kindly donated by owners. We try to remove femoral, renal, mesenteric and pulmonary arteries, and place them in physiologic saline solution (PSS) for storage at 4°C. We have already learned that if this can be done within 1 hour of euthanasia, then some sections of arteries will remain viable for up to 10 days.
In order to compare the extent different arteries constrict or dilate, we have to make sure that we start from the same relative baseline level of stretch for every individual artery. We call this process ‘normalization’. In laboratory species, a fixed normalization factor is used for all the arteries tested. We have shown that in dogs, there is sufficient variation between arteries from different sites that the normalization factor should be calculated afresh for each artery. This is important for ensuring the accuracy and repeatability of all myography studies conducted from now on.
We have identified endothelial dysfunction in all the arterial sites we have looked at so far. Interestingly, the renal artery seems to be the most susceptible, despite it being the easiest artery to collect. From histology of the arteries, and by inducing vasodilation by donating nitric oxide, we have shown that this dysfunction is genuine and not an artefact stemming from collection technique.
Challenges of the project
The main challenge has been recruitment of cases, and we are indebted to the owners who have kindly donated their dogs. A lot of planning has gone into ensuring that the euthanasia experience for both dogs and owners has not been negatively impacted by donation but, as you can imagine, for this type of study where rapid collection is vital to maintaining tissue viability, there has been a great deal of behind-the-scenes work. Since sample collection is relatively non-invasive, dogs can still be individually cremated, if requested. Feedback from owners has been very positive and our setup will form a template for future studies.
Collection of viable pulmonary arteries, which tend to act differently to other arteries, remains elusive, but we are confident of refining our technique to optimize our chances of maintaining viability. Separating the effects of ageing from MMVD is also proving challenging because most of our older dogs have MMVD. We hope to overcome this as we collect samples from more and more dogs.
A significant hurdle we have overcome is the 10-day duration for which arteries can remain viable. This means that if collected rapidly, studies can be extended into the following week. It also opens up the opportunity of collecting arteries from multiple centres, increasing the power of this and future studies.
Applying to BSAVA PetSavers for funding
This work forms part of Marco’s Master’s Degree by Research that was funded by BSAVA PetSavers with additional funding from The Kennel Club Trust. It has allowed him to develop a research career within a clinical environment and was a pivotal first step towards obtaining a PhD studentship. The application process was straightforward and allowed us to pool together some low-level pilot studies, and our data will provide the foundation for future larger grant applications.
We would strongly recommend anyone with a clinical background who is looking to undertake research in academia to consider applying for BSAVA PetSavers funding.
Acknowledgments
We wish also to thank the Histopathology Laboratory of the Easter Bush Pathology Service, Sara-Ann Dickson, Dr. Mary Diaz, Dr. Ian Gow and all the clinical staff of the Hospital for Small Animals of the R(D)SVS involved in patient recruitment.
About the authors
Marco Mazzarella
 Marco graduated from the University of Bologna in 2016. After working in first-opinion small animal practice, he completed a rotating internship and then a cardiology-specific internship at Southfields Veterinary Specialists. In 2021, he undertook a Master of Science by Research, funded by BSAVA PetSavers, within the Roslin Institute at the University of Edinburgh, which he successfully completed with merit in 2022. He is currently studying towards a PhD at the University of Bristol, continuing his work on endothelial dysfunction in canine MMVD.
Marco graduated from the University of Bologna in 2016. After working in first-opinion small animal practice, he completed a rotating internship and then a cardiology-specific internship at Southfields Veterinary Specialists. In 2021, he undertook a Master of Science by Research, funded by BSAVA PetSavers, within the Roslin Institute at the University of Edinburgh, which he successfully completed with merit in 2022. He is currently studying towards a PhD at the University of Bristol, continuing his work on endothelial dysfunction in canine MMVD.
Geoff Culshaw
 An RCVS specialist in Veterinary Cardiology since 2011, Geoff regularly publishes his clinical and lab-based cardiovascular research in both veterinary and basic science journals and has frequently presented his work on national and international stages. He graduated from Glasgow Vet School in 1994 and, after 11 years in general practice, he joined The Royal (Dick) School of Veterinary Studies in 2005 and obtained the RCVS Diploma in Veterinary Cardiology in 2008, and a PhD in 2018. He heads the Small Animal Cardiology Service and is a Clinical Research Associate of the Roslin Institute.
An RCVS specialist in Veterinary Cardiology since 2011, Geoff regularly publishes his clinical and lab-based cardiovascular research in both veterinary and basic science journals and has frequently presented his work on national and international stages. He graduated from Glasgow Vet School in 1994 and, after 11 years in general practice, he joined The Royal (Dick) School of Veterinary Studies in 2005 and obtained the RCVS Diploma in Veterinary Cardiology in 2008, and a PhD in 2018. He heads the Small Animal Cardiology Service and is a Clinical Research Associate of the Roslin Institute.
References
- Buchanan JW (1977) Chronic valvular disease (endocardiosis) in dogs. Advances in Veterinary Science and Comparative Medicine 21, 75-106
- Hadi HA, Carr CS and Al Suwaidi J (2005) Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vascular Health and Risk Management 1, 183–198
- Jones ID, Luis Fuentes V, Boswood A et al. (2012) Ultrasonographic measurement of flow-mediated vasodilation in dogs with chronic valvular disease. Journal of Veterinary Cardiology 14, 203–210
- Moesgaard SG, Klostergaard C, Zois NE et al. (2012) Flow-Mediated Vasodilation Measurements in Cavalier King Charles Spaniels with Increasing Severity of Myxomatous Mitral Valve Disease. Journal of Veterinary Internal Medicine 26, 61–68
- Arribas SM, Hillier C, González C et al. (1997) Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension 30, 1455–1464
- Wenceslau CF, McCarthy CG, Earley S et al. (2021) Guidelines for the measurement of vascular function and structure in isolated arteries and veins. American Journal of Physiology-Heart and Circulatory Physiology 321(1), H77-H111