Beta-adrenergic receptors in canine haemangiosarcoma
10 June 2024


In 2020, Ana Ortiz, Cinzia Allegrucci and Kerstin Baiker were awarded BSAVA PetSavers’ funding to investigate the expression of ß-adrenergic receptors (ß-AR) in canine haemangiosarcoma (HSA). Former master’s student and anatomic pathology resident Kian Guerzoni, and more recent master’s student and anatomic pathology resident Marta Pereira also joined the research team. This study aims to be a baseline investigation towards the potential benefit of using non-selective ß-AR antagonists in the management of canine HSA.
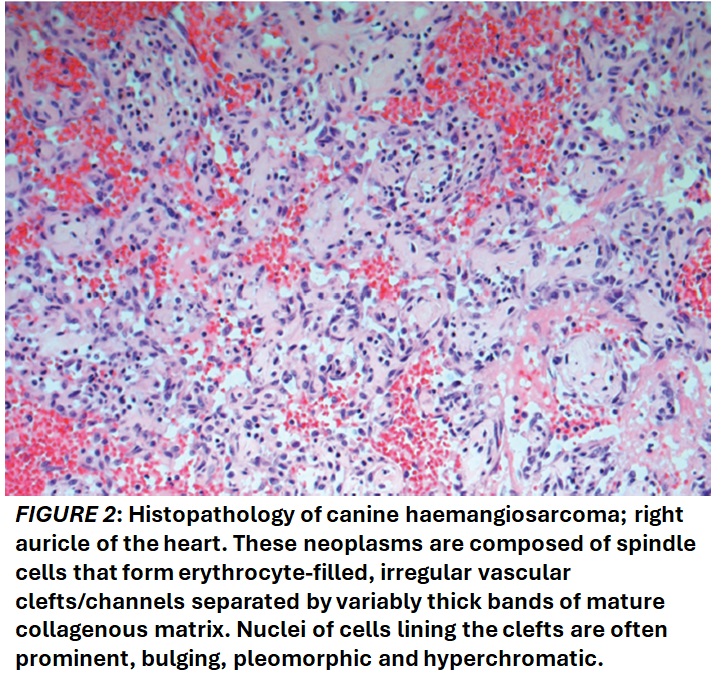
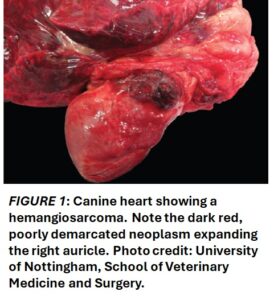 Canine visceral haemangiosarcoma is a malignant vascular tumour, with an hypothesized origin from differentiated vascular endothelial cells or bone marrow haematopoietic stem cells with hemangioblastic potential. It most frequently arises in the spleen or right atrium or auricle of the heart (Figure 1), but it can also present concurrently at multiple sites. On histopathology, the typical morphology consists of pleomorphic spindle cells that form irregular vascular clefts/channels (Figure 2) and/or solid areas of closely packed pleomorphic spindle cells that may form inconspicuous, smaller vascular channels. Canine visceral haemangiosarcoma has a grave prognosis, poor response to conventional therapy and a high metastatic rate. Using current therapeutic approaches of chemotherapy Beta-adrenergic receptors in canine haemangiosarcoma and surgical excision, the median survival time for dogs with visceral HSA stands at approximately 8 months. Human oncology has started targeting ß-ARs across a diverse range of vascular tumours since discovering improvements in therapeutic outcomes by using a non-selective ß-AR antagonist in infantile haemangioma, a benign vascular tumour. The implementation of this novel adjunct therapy to human malignant vascular tumours has led to improved progression-free survival times and median overall survival times.
Canine visceral haemangiosarcoma is a malignant vascular tumour, with an hypothesized origin from differentiated vascular endothelial cells or bone marrow haematopoietic stem cells with hemangioblastic potential. It most frequently arises in the spleen or right atrium or auricle of the heart (Figure 1), but it can also present concurrently at multiple sites. On histopathology, the typical morphology consists of pleomorphic spindle cells that form irregular vascular clefts/channels (Figure 2) and/or solid areas of closely packed pleomorphic spindle cells that may form inconspicuous, smaller vascular channels. Canine visceral haemangiosarcoma has a grave prognosis, poor response to conventional therapy and a high metastatic rate. Using current therapeutic approaches of chemotherapy Beta-adrenergic receptors in canine haemangiosarcoma and surgical excision, the median survival time for dogs with visceral HSA stands at approximately 8 months. Human oncology has started targeting ß-ARs across a diverse range of vascular tumours since discovering improvements in therapeutic outcomes by using a non-selective ß-AR antagonist in infantile haemangioma, a benign vascular tumour. The implementation of this novel adjunct therapy to human malignant vascular tumours has led to improved progression-free survival times and median overall survival times.
 What are ß-ARs?
What are ß-ARs?
For a better understanding of why we have decided to look into these receptors in canine HSA, Marta prepared the following review summary about what these are and their relevance in cancer.
G-coupled ß-AR protein receptors are part of the sympathetic nervous system and are present on many different types of normal human tissues as well as endothelial cells.1 They mediate sympathetic responses in the cardiovascular, pulmonary, and central nervous systems. In the classical signalling pathway, catecholamine binding to ß-AR initiates a signalling cascade that promotes the conversion of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). The cAMP then activates the protein kinase A pathway, which results in the production of different proangiogenic factors, such as vascular endothelial growth factor (VEGF), matrix metalloproteinase 9 (MMP-9) and interleukin (IL)-6.2 VEGF, MMP-9 and IL-6, which stimulate angiogenesis and inflammation, are strongly expressed in HSA, which suggests that the neoplastic cells utilize these factors to modify their microenvironment and stimulate tumour growth.2 This indicates that ß-adrenergic signalling may be an important promoter of growth in these neoplasms.
ß1-AR, ß2-AR, and ß3-AR are expressed in many types of human neoplasms, including malignant vascular tumours.2-4 ß-AR antagonists (beta blockers), such as propranolol, have been correlated with reduced metastasis and cancer progression in many different types of cancer, such as breast cancer,5,6 ovarian cancer,7 melanoma,8 and prostate cancer.9,10
Infantile haemangiomas have been shown to express ß3-AR, ß2-AR, and its phosphorylated form, ß2-ARP.11 Treatment with propranolol, a non-selective ß-AR antagonist (beta blocker), has proved highly effective in infantile angiomas.11-13 Malignant vascular tumours are rarely diagnosed in humans, which limits the development of optimized therapies.2 Because ß-AR are also expressed in malignant vascular tumours, such as angiosarcomas, some research has been conducted into the use of beta blockers in these tumours. All types of ß-AR have been identified in angiosarcomas and haemangioendotheliomas in humans.1,4 A study showed that propranolol helps arrest the cell proliferation and induces apoptosis of neoplastic cells in such human malignant vascular tumours.4 However, the use of propranolol alone did not completely stop neoplastic cell proliferation, and combination with chemotherapy was suggested as the optimal option. This combination of propranolol with chemotherapy has since been further substantiated by numerous studies showing great success in the treatment of human angiosarcomas and haemangioendotheliomas.3,14-20
What are the aims of this research?
To determine whether the therapeutic benefits of ß-AR antagonists as a new therapeutic intervention could be investigated in canine HSA, it is necessary to better understand the expression of these receptors in this neoplasm in the following ways:
1. Using immunohistochemistry, this study will examine where the receptors are expressed and their level of expression in neoplastic tissue.
2. To determine whether there are differences in expression between primary, metastatic neoplastic tissue and matched normal tissue.
What have we learned so far?
Immunohistochemistry protocols have been developed and optimized in canine tissue for anti ß1-AR, ß2-AR, and ß3-AR antibodies. Immunohistochemical staining of the different tissues is currently ongoing, so it is too early to report on findings; however, we hypothesize that:
1. ß-ARs are expressed in canine HSA.
2. There may be inter-individual variability in the expression of the three subtypes of ß-AR genes (ADRB1, ADRB2, ADRB3).
What will be the benefits of this research?
Having as a starting point the characterization of the expression of ß-AR in canine HSA, the long-term objective of this study is to act as a platform for further clinical research into the therapeutic benefits of non-selective ß-AR antagonists in the management of canine HSA.
Thinking about applying for PetSavers’ funding?
PetSavers’ funding made this study possible. While we are very fortunate to have access to tissue samples from a busy veterinary anatomical pathology service receiving both first-opinion and referral cases, the funding allocated to the project allowed the purchase of the necessary antibodies, and covered the costs associated with the immunohistochemical and qPCR analysis. PetSavers is a fantastic institution that supports clinical research with a clear potential positive impact in companion animal health, so applying for PetSavers’ funding comes with a great chance of getting all those great clinical research project ideas out of the drawer and contributing to improved companion animal health. This project allowed the creation of new research collaborations and offered residents an opportunity to participate in a funded research study as part of the research component of their residency training.
The project work is being carried out with exceptional technical support that we would like to acknowledge: for the immunohistochemistry, Histology Technical Specialist Emma Pritchard, and we would also like to acknowledge PhD student Megan Wilde for her assistance with antibody validation in western blotting, and the Pathology team for their assistance with sample collection.
Please support us in our 50th year to continue this life changing work for pets by visiting the donation page on our website: https://www.bsava.com/petsavers/donate/
